Self-healable polymer gels with multi-responsiveness of gel–sol–gel transition and degradability†
Abstract
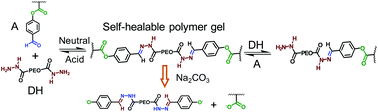
“Self-healable” materials can recover their structures and functions upon injury, which is extremely attractive for emerging biomedical applications. In this research, P(N-isopropyl acrylamide-co-4-formylphenyl acrylate) P(NIPAM-co-FPA) with a pendant benzaldehyde group though connection with a base-labile phenolic ester bond was designed, and polymer gels were prepared with PEO dihydrazide as a cross-linker. The polymer gels formed automatically by self-catalysis but their self-healing property was dependant on cross-linking density. The self-healing property of the polymer gel improved with decreasing cross-linking density based on decreasing FPA composition, increasing molecular weight of the cross-linker, and decreasing gelator concentration. Based on the reversibility of the acylhydrazone bond, the polymer gel showed gel–sol–gel transition by variation of acidity and group ratios. Furthermore, the gels could be degraded by Na2CO3 solutions because the phenolic ester bond is base-labile.


 Please wait while we load your content...
Please wait while we load your content...