The para-fluoro-thiol ligation in water†
Abstract
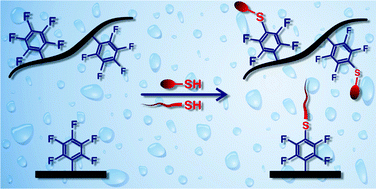
The para-fluoro-thiol reaction (PFTR) is performed for the first time in aqueous media. A water-soluble model scaffold based on poly(N,N-dimethylacrylamide) and incorporating pentafluorostyrene units was synthesized by nitroxide-mediated copolymerization. The effect of pH, temperature, and stoichiometry on the solution-phase kinetics of the PFTR for side-chain modification is studied by 19F and 1H NMR spectroscopy using mercaptoethanol as a model thiol. While the reaction can occur from pH 11 on, increasing pH greatly accelerates the reaction. Yields >90% are achieved at pH 12 and 40 °C in 24 h, while quantitative coupling is reached at pH 13 and 50 °C in less than 16 h. Grafting of a cysteine-containing peptide on tissue culture plates demonstrated by ToF-SIMS evidences the benefit of performing this reaction in aqueous media for surface functionalization of materials prone to degradation in organic solvents.


 Please wait while we load your content...
Please wait while we load your content...