Chiral anion-triggered helical poly(ionic liquids)†
Abstract
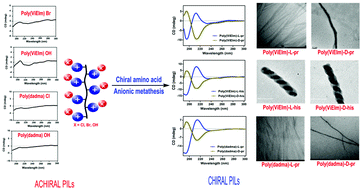
Synthetic chiral polymers and helical polymers are finding increasing applications in catalysis and chiral recognition. Herein, we induced chirality in the PILs at the anionic site by introducing optically pure amino acids via a simple anionic exchange reaction. RAFT-derived imidazolium-based poly(ionic liquids) [poly(ViEIm) Br] and the commercially available poly(diallyldimethylammonium chloride) were utilized for the synthesis of six different chiral PILs (poly(ViEIm)-L-pr, poly(ViEIm)-D-pr, poly(ViEIm)-L-his, poly(ViEIm)-D-his, poly(dadma)-L-pr and poly(dadma)-D-pr). The synthesized chiral poly(ionic liquids) (CPILs) were well characterized via NMR, DSC, TGA, specific rotation, CD, SEM and TEM analyses. Chiroptical studies suggested that introduction of chiral anions at the anionic site of achiral PIL-Br/OH will trigger helicity. These CPILs possess tuneable chirality as a function of the anion. The chiral PILs possessing L- and D-amino acids as counter anions showed completely opposite helical loops in CD studies and opposite specific rotation values. The morphological studies carried out via SEM and TEM clearly showed the existence of a random double helix nature for some of the synthesized chiral PILs. The synthesized CPILs were further used as catalysts for the Baylis–Hillman reaction between methyl vinyl ketone and aryl aldehydes that gave B–H product up to 90% with moderate enantiomeric excess.


 Please wait while we load your content...
Please wait while we load your content...