A PHEA-g-PEO well-defined graft copolymer exhibiting the synchronous encapsulation of both hydrophobic pyrene and hydrophilic Rhodamine 6G†
Abstract
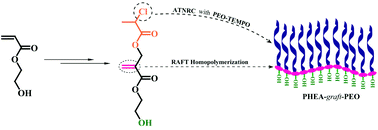
A well-defined graft copolymer consisting of a poly(2-hydroxyethyl acrylate) (PHEA) backbone and poly(ethylene oxide) (PEO) side chains was synthesized by successive reversible addition–fragmentation chain transfer (RAFT) polymerization and atom transfer nitroxide radical coupling (ATNRC) reaction. RAFT homopolymerization of a Cl-containing acrylate monomer, 2-hydroxyethyl 2-((2-chloropropanoyloxy)methyl)acrylate (HECPMA), was first performed in a controlled way to afford a well-defined PHEA-based backbone with a low polydispersity (Mw/Mn = 1.08). The target poly(2-hydroxyethyl acrylate)-g-poly(ethylene oxide) (PHEA-g-PEO) graft copolymer with a narrow molecular weight distribution (Mw/Mn = 1.16) was then obtained by ATNRC reaction between the pendant –OCOCH(CH3)Cl group in every repeated unit of PHEA-based backbone and PEO with a TEMPO end group via the grafting-onto strategy, using CuCl/PMDETA as a catalytic system. The critical micelle concentrations (cmcs) of the obtained graft copolymer in pure water, brine, and aqueous Na2SO4 solution were determined by the fluorescence probe technique using N-phenyl-1-naphthylamine as probe and micellar morphologies in aqueous media were visualized by TEM. It was found that PHEA-g-PEO graft copolymer self-assembled into large compound micelles in aqueous media, which can encapsulate hydrophilic Rhodamine 6G and hydrophobic pyrene separately or simultaneously.

 Please wait while we load your content...
Please wait while we load your content...