1,3-Dipolar and Diels–Alder cycloaddition reactions on polyester backbones possessing internal electron-deficient alkyne moieties†
Abstract
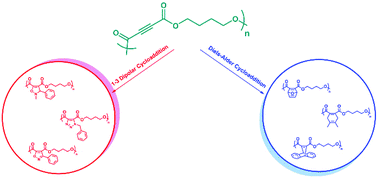
In this study we prepared a series of polyesters containing electron deficient internal alkyne units derived from acetylene dicarboxylic acid in the main backbone. Next, one of the polyesters was employed as a polymeric platform in copper free cycloaddition reactions like, Huisgen type 1,3-dipolar and Diels–Alder cycloaddition reactions in the presence of various dipoles and dienes, respectively. The 1,3-dipolar cycloaddition reactions were performed at mild temperatures (rt and 40 °C) in 1,4-dioxane for 16 h and had moderately high efficiencies (80–100%). However, the Diels–Alder cycloaddition reactions were carried out at higher temperatures (60 to 120 °C) in 1,4-dioxane for 16 h with reasonable efficiencies (45–97%). Moreover, the polyester was successfully used in one-pot 1,3-dipolar, sequential 1,3-dipolar/Diels–Alder, and sequential 1,3-dipolar cycloaddition/retro-Diels–Alder reactions.


 Please wait while we load your content...
Please wait while we load your content...