Metal-free RAFT cationic polymerization of p-methoxystyrene with HCl·Et2O using a xanthate-type RAFT cationogen†
Abstract
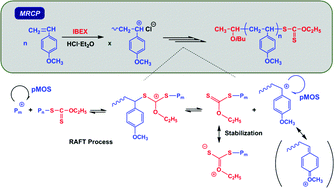
The metal-free RAFT cationic polymerization (MRCP) of p-methoxystyrene (pMOS) was achieved. It was demonstrated that O-ethyl S-(1-isobutoxyethyl) carbonodithioate (IBEX) efficiently acted as a RAFT agent for cationic polymerization, i.e. as a RAFT cationogen, initiated with HCl·Et2O with good control over the molecular weight, polydispersity, and chain ends of the resulting poly(pMOS). However, when using similar RAFT cationogens such as dithioester- or acetate-types that are available for the MRCP of vinyl ethers with HCl·Et2O, no or uncontrolled polymerization proceeded, respectively. In the case of the polymerization of pMOS using HCl·Et2O in the absence of a RAFT cationogen, the polymerization was uncontrolled. From the results of the copolymerization of pMOS and vinyl ether, the key to success in the controlled cationic polymerization of pMOS is to utilize a carbocation generated on the vinyl ether in the initiation stage. Through the end-functionality analysis and the model reaction of RAFT cationogens with HCl·Et2O, IBEX was shown to act via the degenerative addition–fragmentation of cationic species, and therefore undergo polymerization via the so-called RAFT mechanism. The resulting poly(pMOS) obtained by this MRCP could be used as a macro-chain transfer agent for RAFT radical polymerization to synthesize novel block copolymers such as poly(pMOS)-b-poly(vinyl acetate).


 Please wait while we load your content...
Please wait while we load your content...