Highly stable electrochromic and electrofluorescent dual-switching polyamide containing bis(diphenylamino)-fluorene moieties†
Abstract
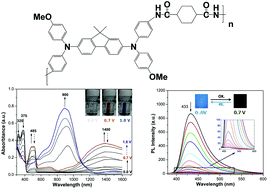
Two kinds of electroactive polyamides with bis(diphenylamino)-fluorene units acting as electroactive fluorophores were prepared via condensation polymerization from a novel diamine, N,N′-bis(4-aminophenyl)-N,N′-bis(4-methoxyphenyl)-2,7-diamino-9,9-dimethylfluorene, with cycloaliphatic and aromatic dicarboxylic acids, respectively. These polyamides exhibited excellent solubility in various organic solvents and outstanding thermal stability. Cyclic voltammograms of the polyamide films revealed two reversible redox couples with half-wave potentials at 0.54–0.56 and 0.75–0.78 V, respectively. The polyamide films showed excellent reversible stability of multicolor electrochromic characteristics (colorless-orange-blue) after over 1000 cyclic switches. Compared with the polyamide derived from aromatic dicarboxylic acids, the polyamide derived from cycloaliphatic dicarboxylic acid exhibited strong fluorescence with quantum yield up to 50.2%. Furthermore, the fluorescence could be reversibly modulated by electrochemical redox with superior stability and a high contrast ratio of 152.

 Please wait while we load your content...
Please wait while we load your content...