Non-covalent interactions in controlling pH-responsive behaviors of self-assembled nanosystems†
Abstract
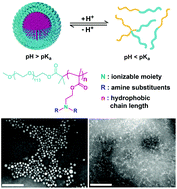
Self-assembly and associated dynamic and reversible non-covalent interactions are the basis of protein biochemistry (e.g., protein folding) and the development of sophisticated nanomaterial systems that can respond to and amplify biological signals. In this study, we report a systematic investigation on non-covalent interactions that affect the pH responsive behaviors and the resulting supramolecular self-assembly of a series of ultra-pH sensitive (UPS) block copolymers. An increase of hydrophobic and π–π stacking interactions led to a decrease of pKa values. In contrast, enhancement of direct ionic binding between cationic ammonium groups and anionic counter ions gave rise to increased pKa. Moreover, hydration of hydrophobic surfaces and hydrogen bonding interactions may also play a role in the self-assembly process. The key parameters capable of controlling the subtle interplay of different non-covalent bonds in the pH-triggered self-assembly of UPS copolymers are likely to offer molecular insights to understand other stimuli-responsive nanosystems. Selective and precise implementation of non-covalent interactions in stimuli-responsive self-assembly processes will provide powerful and versatile tools for the development of dynamic, complex nanostructures with predictable and tunable transitions.

 Please wait while we load your content...
Please wait while we load your content...