Enantio- and diastereoselective polymerization: asymmetric allylic alkylation catalyzed by a planar-chiral Cp′Ru complex†
Abstract
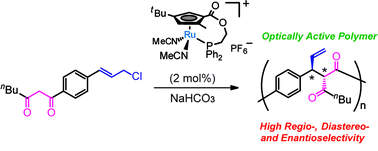
In this study, we examined the regio-, diastereo-, and enantioselective polymerization using asymmetric allylic alkylation catalyzed by a planar-chiral cyclopentadienyl-ruthenium (Cp′Ru) complex ((S)-1). Achiral AB type monomers bearing 1,3-diketone and allylic chloride moieties were polymerized by (S)-1 with quantitative monomer conversion. The resulting polymer (3) was characterized by NMR analyses; the polymerization reaction catalyzed by (S)-1 proceeded with high regioselectivity, and the vicinal stereocenters of the monomer unit can be controlled. The polymer showed a Cotton effect at around 270 nm in its CD spectrum, which was ascribed to the bisignate coupling between the π–π* transition moment of each benzene chromophore. The results suggested that the polymerization proceeded in a stereoselective manner to give optically active polymers.

 Please wait while we load your content...
Please wait while we load your content...