σ-p Conjugated copolymers via dehydrocoupling polymerization of phenylsilane and mesitylborane†
Abstract
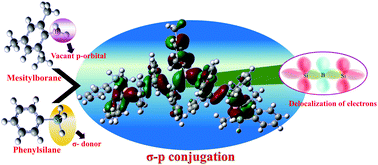
There are a number of π-conjugated organoboron polymers in which π-conjugation is extended via the vacant p-orbital of the boron atom. However, boron incorporation into σ-conjugated systems has not been reported so far. A novel σ-p conjugated copolymer of phenylsilane and mesitylborane was synthesized by dehydrocoupling polymerization using a rhodium catalyst. Change in electronic states due to the incorporation of the boron moiety was determined both by DFT calculations and experiments. The obtained colorless polymers were characterized by 1H-NMR and 11B-NMR spectra. Incorporation of boron was confirmed by 11B-NMR, while the Si/B ratio was calculated by 1H-NMR integration ratios. GPC analysis showed the Mn of the polymer as 1200–2900 g mol−1. The copolymers showed high sensitivity towards fluoride ions both optically and electrochemically exhibiting a “turn on” type of sensing mechanism.

 Please wait while we load your content...
Please wait while we load your content...