Analyzing the discrepancies in the activation energies of the backbiting and β-scission reactions in the radical polymerization of n-butyl acrylate
Abstract
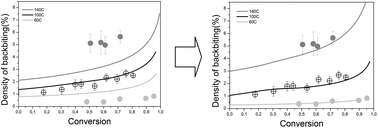
The formation of midchain radicals (MCRs) and their subsequent fate heavily influences the radical polymerization of acrylic monomers. This article aims to shed light on the discrepancies in the activation energies of the formation of MCRs by backbiting and their reaction by beta-scission in the radical polymerization of n-BA determined by both experimental and theoretical methods. For this purpose, bulk and solution batch polymerizations of n-BA at different solids contents and over a wide range of nominal temperatures of 60, 100 and 140 °C initiated by a thermal initiator were carried out and the kinetics, branching density and average molar masses as well as the macromonomer density were measured by means of 13C NMR, 1H NMR and size-exclusion chromatography with multi-angle light scattering (SEC/MALS). A detailed model was used to predict the experimental data and to estimate the activation energies for the backbiting and β-scission reactions. The estimated values for the backbiting activation energy are closer to the upper range of values previously reported and, on the other hand, the estimation shows that in the whole range of temperatures, the β-scission rate coefficient should be higher than the accepted values reported in the literature which failed to predict the properties in the wide range of temperatures used in this work.

 Please wait while we load your content...
Please wait while we load your content...