Tripodal hydrogen bond donor binding with sulfonic acid enables ring-opening polymerization†
Abstract
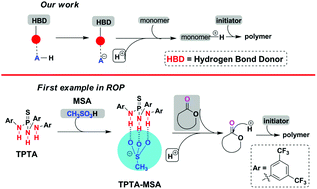
H-bond donor (HBD) and Brønsted acid (BA) co-catalysis (HBD–BA) performed ring-opening polymerization (ROP) of cyclic esters in solution at room temperature. The HBD–BA strategy using thiophosphoric triamide (TPTA) binding with methanesulfonic acid (MSA) enabled the ROP of L-lactide (LA) to polylactide (PLA) with predicted molecular weights (Mn = 2.9–13.8 kDa) and low dispersities (Đ = 1.18–1.22). Associations of TPTA with MSA through triple hydrogen bonds stabilized the sulfonate anion and enhanced the catalytic performance of TPTA–MSA. Homopolymers of LA, trimethylene carbonate (TMC), δ-valerolactone, and ε-caprolactone (CL), and diblock copolymers PLA-b-PTMC and PLA-b-PCL with predicted Mn and narrow Đ were synthesized. NMR measurements, kinetics investigations, and chain extension experiments indicated that the TPTA–MSA catalyzed ROPs were controlled/living in nature. This is the first Brønsted acidic catalysis platform workable in all of the three major types of cyclic ester monomers including lactides, cyclic carbonates, and lactones.


 Please wait while we load your content...
Please wait while we load your content...