Tuning oxygen permeability in azobenzene-containing side-chain liquid crystalline polymers†
Abstract
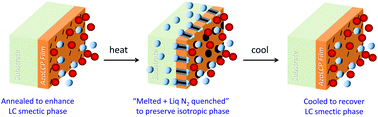
A series of poly[4-(4-cyanoazobenzene-4′-oxy)alkyl methacrylate]s, side-chain liquid crystalline polymers (azoLCPs) were synthesized with methylene groups as spacers varying from 5 to 12. The thermal properties and phase transition temperatures of the polymers were characterized with differential scanning calorimetry and polarized optical microscopy and a relationship between spacer lengths, glass transition (Tg) and clearing temperatures (Tc) of the polymers was established. The Tg decreased with increasing spacer length, while the Tc exibited an odd–even effect with varying spacer length. X-ray diffraction was used to determine the degree crystallinity above and below Tc. The azoLCPs exhibited smectic ↔ nematic ↔ isotropic phases. Increasing crystallinity correlated linearly with decreasing gas permeability as measured using an oxygen permeation analyzer, which was used to measure the films’ permeability to oxygen (O2) gas. Switching of the azoLCPs from a liquid crystalline to an isotropic state was accomplished by heating the films above their Tc, which resulted in at least a 10-fold increase in the O2 permeability coefficient (PO2). Increasing the methylene spacer length of the azoLCP had little or no effect on gas permeability however it did decrease the Tc, allowing fine control of the temperature at which the switch in PO2 takes place by tuning the mesophase between nematic and isotropic states.

 Please wait while we load your content...
Please wait while we load your content...