pH-Responsive non-ionic diblock copolymers: protonation of a morpholine end-group induces an order–order transition†
Abstract
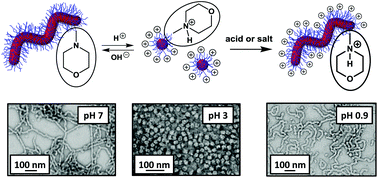
A new morpholine-functionalised, trithiocarbonate-based RAFT agent, MPETTC, was synthesised with an overall yield of 80% and used to prepare a poly(glycerol monomethacrlyate) (PGMA) chain transfer agent. Subsequent chain extension with 2-hydroxypropyl methacrylate (HPMA) using a RAFT aqueous dispersion polymerisation formulation at pH 7.0–7.5 resulted in the formation of morpholine-functionalised PGMA-PHPMA diblock copolymer worms via polymerisation-induced self-assembly (PISA). These worms form soft, free-standing aqueous hydrogels at 15% w/w solids. Acidification causes protonation of the morpholine end-groups, which increases the hydrophilic character of the PGMA stabiliser block. This causes a subtle change in the copolymer packing parameter which induces a worm-to-sphere morphological transition and hence leads to in situ degelation at pH 3. This order–order transition was characterised by dynamic light scattering, transmission electron microscopy and gel rheology studies. On returning to pH 7, regelation is observed at 15% w/w solids, indicating the reversible nature of the transition. However, such diblock copolymer worm gels remain intact when acidified in the presence of electrolyte, since the terminal cationic charge arising from the protonated morpholine end-groups is screened under these conditions. Moreover, regelation is also observed in relatively acidic solution (pH < 2), because the excess acid acts as a salt under these conditions and so induces a sphere-to-worm transition.

 Please wait while we load your content...
Please wait while we load your content...