One-pot catalyst-free synthesis of down- and upconversion fluorescent oligopyrazolines from diazoacetates and maleic anhydride†
Abstract
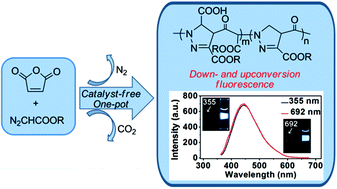
Conventionally, each fluorophore is only illuminated with the wavelength of incident light shorter (downconversion) or longer (upconversion) than that of emitted light. However, the oligopyrazolines presented in this paper are the down- and upconversion fluorescent molecules with a maximum emission at 445 nm, which are facilely synthesized from diazoacetates and maleic anhydride (MA) without any catalyst by a newly developed one-pot process. The intermediates of oligopyrazolines are formed through 1,3-dipolar cycloaddition and a cascade ring-opening insertion, which transform into oligomers in situ with molecular weight around 2 × 103 g mol−1. The structures of the subunits and resulting oligopyrazolines are clarified clearly by MALDI-TOF-MS and the quantum yield with excitation at 350 nm is in the range of 12–19%. A cell experiment with HeLa cells demonstrates its promising potential for bioimaging applications.

 Please wait while we load your content...
Please wait while we load your content...