Entropically-driven ring-opening metathesis polymerization (ED-ROMP) of macrocyclic olefins prepared from deoxycholic acid to give functionalized polymers†‡
Abstract
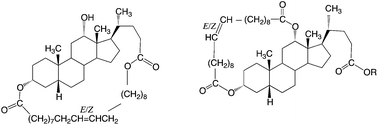
Three new families of macrocyclic olefins were prepared from deoxycholic acid. Two of these have a novel structure in that the bile acid unit is linked to a C20 fatty acid unit via the 3α- and 12α-positions, thus creating macrocycles with rings with a repeat unit of 29 ring atoms. Entropically-driven ring-opening methathesis polymerizations (ED-ROMPs) of the macrocycles gives polymers with free hydroxyl groups, or free methyl or t-butyl carboxylic ester groups. The latter could be converted into carboxylic acid groups by treatment with trifluoroacetic acid. The polymers form transparent hole-free films that are potentially useful for supporting cell growth.
- This article is part of the themed collection: David Sherrington Commemorative Issue

 Please wait while we load your content...
Please wait while we load your content...