Mild Brønsted acid initiated controlled polymerizations of 2-oxazoline towards one-pot synthesis of novel double-hydrophilic poly(2-ethyl-2-oxazoline)-block-poly(sarcosine)
Abstract
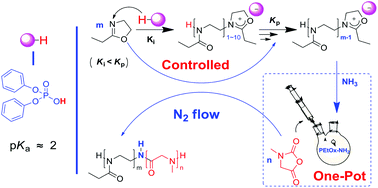
Cationic ring-opening polymerization (CROP) of 2-oxazolines is initiated with acylation, alkylation, and silylation on the 2-oxazoline nitrogen. A mild acid initiator for CROP of 2-oxazoline has never been reported. Here we demonstrated that a mild Brønsted acid, diphenyl phosphate (DPP), initiated controlled CROP of 2-ethyl-oxazoline (EtOx) towards PEtOx and its diblock copolymers. PEtOx, with Mw ranging from 3000 to 15 000 g mol−1, and narrow dispersities (Đ ≤ 1.13), was successfully produced by feed molar ratios. The kinetics plot showed that the polymerization was in a two stage mode with a marked acceleration in rate at the early stage. Moreover, the propagation during the late stage proceeded quickly (3.73 × 10−4 L mol−1 s−1). NMR investigations supported a cationic nature of the initiation with DPP. Novel double-hydrophilic poly(2-ethyl-2-oxazoline)-block-poly(sarcosine) diblock copolymers were synthesized via a one-pot two-step approach.

 Please wait while we load your content...
Please wait while we load your content...