Trehalose hydrogels for stabilization of enzymes to heat†
Abstract
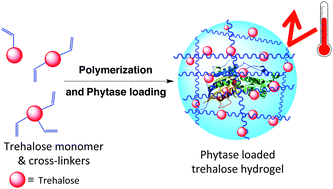
Enzymes can catalyze various reactions with high selectivity and are involved in many important biological processes. However, the general instability of enzymes against high temperature often limits their application. To address this, we synthesized a trehalose-based hydrogel in two steps from commercial starting materials with minimal purification procedures. Mono- and multi-functional trehalose monomers were cross-linked by redox-initiated radical polymerization to form a hydrogel. Phytase, an important enzyme utilized in animal feedstock, was employed to study the effectiveness of the trehalose hydrogel to stabilize proteins against heat. Addition of the phytase solution to the hydrogel resulted in enzyme internalization as confirmed by confocal microscopy. The phytase in the hydrogel retained 100% activity upon heating at 90 °C compared to 39% when the hydrogel was absent. The enzyme could also be recovered from the hydrogel. The trehalose hydrogel synthesis reported herein should be readily scalable for thermal stabilization of a wide variety of enzymes.

 Please wait while we load your content...
Please wait while we load your content...