Well-controlled and stable emulsion ATRP of MMA with low surfactant concentration using surfactant–ligand design as the copper capture agent†
Abstract
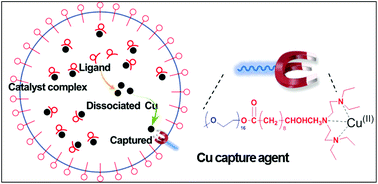
In emulsion atom transfer radical polymerization (ATRP), escape of catalytic copper ion species from the particle to the aqueous phase represents one of the major challenges which causes uncontrollable polymerization and/or unstable latex. We addressed this issue by employing a functional surfactant–ligand (SL) design as the capture agent (CA), which prevented the copper species from escape in the emulsion ATRP. In this work, emulsion ATRP of methyl methacrylate (MMA) mediated CuCl2/4,4-di(5-nonyl)-2,2′-bipyridine (dNbpy) was carried out with Brij-98 as a surfactant in the presence of a small amount of CA. The CA molecules effectively captured dissociated copper ions inside the particle, thus efficiently decreasing the copper ion concentration in the aqueous phase. Only 3 wt% of surfactant loading (0.6 wt% CA and 2.4 wt% Brij-98 based on MMA) was needed to achieve well-controlled ATRP and to stabilize the emulsion system, which is a significant reduction from a typical 13–18 wt% often used in the studies reported in the literature.

 Please wait while we load your content...
Please wait while we load your content...