A novel biodegradable polymeric surfactant synthesized from carbon dioxide, maleic anhydride and propylene epoxide†
Abstract
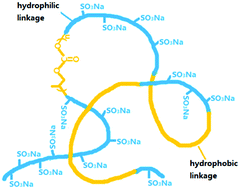
Terpolymerizations of CO2, propylene epoxide (PO) and maleic anhydride (MA) using zinc adipate (ZnAA) as a catalyst were carried out in a toluene solution. A series of biodegradable terpolymers (PPCMAs) with different polyester and polycarbonate contents were synthesized. The molecular chain sequence structure of these terpolymers was proved to be a gradient one based on 1H NMR investigation combined with in situ infrared technology monitoring the reaction process. The sulfonation of biodegradable PPCMAs was carried out by the addition of sodium hydrogen sulphite into ethylenic double bonds of the unsaturated polyester unit. The surface activities and the aggregation of these sulfonated biodegradable terpolymers in aqueous solution were investigated by surface tension measurement and dynamic light scattering (DLS) technology. The results indicate that the sulfonated biodegradable terpolymer with comparable hydrophilic and hydrophobic segment contents tends to adsorb at the air/water interface and thus exhibits the best surface activities. The surface tension of the aqueous solution of the polymer with 56.8% hydrophilic segments reaches 47.5 mN m−1 at its critical micelle concentration (CMC).

 Please wait while we load your content...
Please wait while we load your content...