Hypercrosslinked porous polycarbazoles via one-step oxidative coupling reaction and Friedel–Crafts alkylation†
Abstract
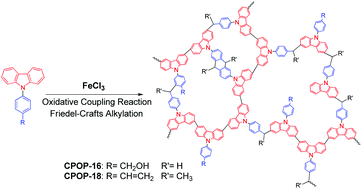
Considering that carbazolyl groups can be coupled through FeCl3-promoted oxidative coupling reaction and vinyl/hydroxymethyl-carrying phenyl groups can be linked by FeCl3-catalyzed Friedel–Crafts alkylation, a facile method for the preparation of hypercrosslinked carbazole-based porous organic polymers (CPOP-16–19) via FeCl3-promoted one-step oxidative coupling reaction and Friedel–Crafts alkylation from the vinyl or hydroxymethyl functionalized carbazole monomers is reported. The Brunauer–Emmett–Teller specific surface area of the obtained polymers is up to 1130 m2 g−1, which is comparable to the other reported hypercrosslinked porous polymers. From their gas adsorption isotherms, the hydrogen uptake capacity of CPOP-19 is high up to 2.39 wt% at 1.0 bar and 77 K, and the uptake capacity for carbon dioxide of CPOP-19 can reach 16.7 wt% at 1.0 bar and 273 K. Moreover, the adsorption capacity of the obtained materials for poisonous and harmful organic vapors such as toluene and formaldehyde was also investigated. The adsorbed amount of toluene by CPOP-19 is 672 mg g−1 (about 7.3 mmol g−1) at its saturated vapor pressure. Among the prepared polymers, CPOP-19 possesses an excellent adsorption capacity for formaldehyde (11.2 mg g−1) under ambient conditions and exhibits good repeatability at the same time, which suggests its potential application in the removal of harmful small molecules from the environment.

 Please wait while we load your content...
Please wait while we load your content...