The intensively enhanced conductivity of polyelectrolytes by amphiphilic compound doping†
Abstract
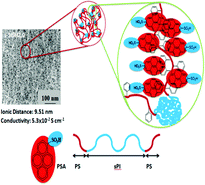
As pyrenesulfonic acid (PSA) is added to sulfonated poly(styrene-b-isoprene-b-styrene) (s-SIS), a novel effect on the conductivity of polyelectrolytes results. In this study, s-SIS was sulfonated to two different degrees to obtain s-SISL (ion-exchange capacity (IEC): 0.92) and s-SISH (IEC: 1.20). After PSA was added to the s-SISL, the proton conductivity increased significantly and reached an optimum at a PSA addition of 4% (s-SISL4.0), where the conductivity (0.6 × 10−2 S cm−1) was 6.0 times higher than s-SISL without PSA (0.1 × 10−2 S cm−1). Similarly, when PSA was added to s-SISH (IEC: 1.20), the proton conductivity achieved an optimum at a PSA addition of 2% (s-SISH2.0), at which point the proton conductivity (5.3 × 10−2 S cm−1) was 26 times that of s-SISH without PSA (0.2 × 10−2 S cm−1). From the TEM analyses of the s-SISH membrane in the presence of different PSA concentrations, this doping PSA effect can be interpreted by means of the interaction between the aromatic ring on the PSA and the aromatic ring on the hydrophobic polystyrene segments of the s-SIS block copolymer. This effect of doping an amphiphilic compound to polyelectrolytes to intensify the conductivity can be widely applied to numerous kinds of polyelectrolytes.

 Please wait while we load your content...
Please wait while we load your content...