Branched polythiophenes by Ni-catalyzed Kumada coupling†
Abstract
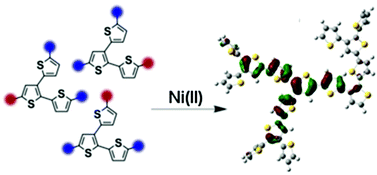
The controlled synthesis of branched polythiophenes via Ni-catalyzed Kumada type coupling of branched AB2 monomers prepared in situ from 5,5′′-dibromo-2,2′:3′,2′′-terthiophene (3TBr2) and 5,5′,5′′-tribromo-2,2′:3′,2′′-terthiophene (3TBr3) is presented. The access to highly branched polymer architectures with rather high molecular weights is a key benefit of this one-pot polymerization with respect to standard oxidative polymerization procedures. Our synthetic route further allows the in situ endgroup functionalization as shown exemplarily for pentene functionalized branched polythiophenes and eases thereby the access to branched polythiophenes with tailor-made properties. In particular a comparison of the optical properties allows conclusions about the architecture of the obtained materials with respect to linear and fully branched systems. Energy levels are obtained by cyclic voltammetry measurements in thin films. DFT calculations provide further guidance on the interpretation of the possible molecular structure of the polymer (i.e. branching density) in correlation with their properties.

 Please wait while we load your content...
Please wait while we load your content...