Synthesis of cationic poly((3-acrylamidopropyl)trimethylammonium chloride) by SARA ATRP in ecofriendly solvent mixtures†
Abstract
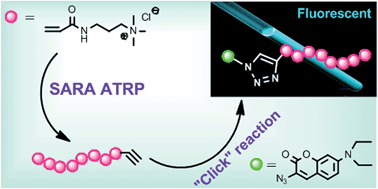
Supplemental activator and reducing agent atom transfer radical polymerization (SARA ATRP) of the cationic monomer (3-acrylamidopropyl)trimethylammonium chloride (AMPTMA) was successfully performed for the first time. The polymerizations were performed in water or ethanol–water mixtures at room temperature in the presence of Cu(0), using relatively low concentrations of soluble copper catalyst and an excess of ligand (Me6TREN). The reaction conditions were optimized to give the best control over the polymerization under environmentally friendly conditions. The polymerization data showed good control over the molecular weights with narrow molecular weight distributions for the entire polymerization. The preservation of the chain-end functionality was confirmed by self-chain extension and the synthesis of a block copolymer containing AMPTMA and oligo(ethylene oxide) methyl ether acrylate (OEOA). SARA ATRP was also extended to the synthesis of alkyne-terminated poly-AMPTMA (PAMPTMA), which was subsequently functionalized, using copper(I) catalyzed azide–alkyne cycloaddition, with an azido-functionalized coumarin derivative.
- This article is part of the themed collection: Polymer Chemistry Lectureship Winners

 Please wait while we load your content...
Please wait while we load your content...