Zirconocene-catalyzed stereoselective cyclocopolymerization of 2-methyl-1,5-hexadiene with propylene†
Abstract
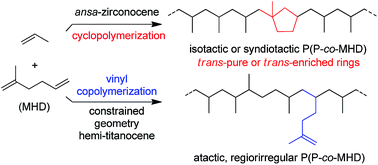
The copolymerization of 2-methyl-1,5-hexadiene (MHD) with propylene has been studied with different single-site group 4 metal catalysts. Systems based on ansa-zirconocene precursors such as rac-{Me2Si(2-Me-4-Ph-Ind}ZrCl2 (1) and C1- or CS-symmetric ansa-{CpCR2Flu}ZrCl2 (2 and 3, respectively), once activated by MAO, are highly active (20–600 kgpol gcat−1 h−1 at 60–70 °C) and yield copolymers in which MHD is cyclopolymerized as methylene-(1-methyl)-1,3-cyclopentane (MMCP) units. 13C NMR studies revealed, depending on the symmetry of the precatalyst used, either highly isotactic (1, 2) or syndiotactic (3) polypropylene (PP) backbones, with isolated MMCP units. Fully trans-diastereoselective cyclopolymerization of MHD was observed with 1/MAO, while a mixture of trans and cis MMCP rings was observed with 2 and 3/MAO. The amount of MMCP units in PP (0.2–1.6 mol%) can be controlled by the amount of MHD in the feed. In contrast, the constrained geometry catalyst system based on {C5Me4SiMe2NtBu}TiCl2 (4) and MAO showed a much lower productivity (ca. 3 kgpol gcat−1 h−1 at 60 °C) and yielded a regioirregular, atactic copolymer in which MHD is simply vinyl-inserted in quite moderate amounts (0.2 mol%).

 Please wait while we load your content...
Please wait while we load your content...