Zwitterionic polymerization of glycidyl monomers to cyclic polyethers with B(C6F5)3†
Abstract
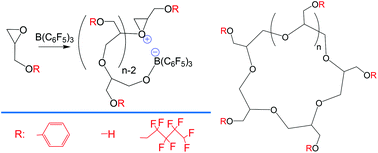
A new method of generating cyclic polyethers is reported. Glycidyl monomers react with B(C6F5)3 to generate cyclic polyethers under anhydrous conditions. In the presence of water, linear chains are formed. A zwitterionic ring-opening polymerization mechanism is postulated based on experimental evidence and DFT calculations. The obtained cyclic polyethers can be considered a new family of crown ethers, where peripheral functional groups such as phenyls, fluorinated aliphatic chains or hydroxyls decorate the rings.

 Please wait while we load your content...
Please wait while we load your content...