Synthesis and polymerization of cyclobutenyl-functionalized polylactide and polycaprolactone: a consecutive ROP/ROMP route towards poly(1,4-butadiene)-g-polyesters†
Abstract
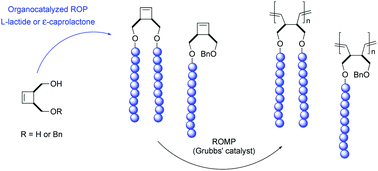
Efficient preparation of cyclobutenyl end-functionalized polyester macromonomers bearing polylactide (PLA) or poly(ε-caprolactone) (PCL) arms was achieved by organocatalyzed ring-opening polymerization (ROP) of L-lactide or ε-caprolactone in the presence of cis-3,4-bis(hydroxymethyl)cyclobutene or cis-4-benzyloxymethyl-3-hydroxymethylcyclobutene acting as an initiator. Cyclobutenyl end-functionalized PLA and PCL macromonomers having one or two arms were obtained in high yields with excellent control over molecular weights (up to 11 000 g mol−1) and dispersity (PDI < 1.25) by organocatalyzed ROP using 4-(N,N-dimethylamino)pyridine (DMAP) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), respectively. Ring-opening metathesis polymerization (ROMP) of the macromonomers using ruthenium-based Grubbs' second generation catalyst afforded well-defined polybutadiene-g-polyester copolymers having an exclusively linear polybutadiene backbone with a strictly 1,4-type microstructure, with molecular weights ranging from 20 000 to 170 000 g mol−1 and low dispersities (PDI ≤ 1.30). The products resulting from this consecutive ROP/ROMP route represent the first examples of poly(1,4-butadiene)-g-polyesters through the macromonomer route.

 Please wait while we load your content...
Please wait while we load your content...