Click chemistry as a powerful and chemoselective tool for the attachment of targeting ligands to polymer drug carriers†
Abstract
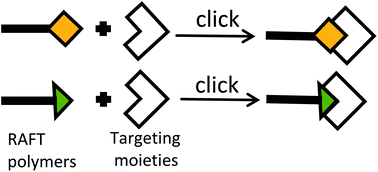
Various click chemistry azide–alkyne cycloaddition reactions were used to attach azide group-terminated peptides to polymer drug carriers in an effort to conjugate biologically active molecules with polymer drug carriers by directly binding unprotected peptides to these polymers. Three methods using click chemistry to conjugate the azide group-containing molecules with synthetic polymers were compared: (1) click chemistry with a Cu(I) catalyst in aqueous and organic solvents, (2) click reactions using ruthenium complex catalysts in DMF and (3) metal-free click chemistry based on a dibenzocyclooctyne (DBCO) reactive group. The suitability of these reactions was verified for the non-covalent attachment of targeting moieties to these polymer carriers via peptide–peptide interactions. Moreover, RAFT polymerization was suggested for the synthesis of semitelechelic copolymers containing a single DBCO group at the polymer chain end and for the preparation of well-defined diblock copolymer drug carriers consisting of specific peptide and hydrophilic polymer blocks.

 Please wait while we load your content...
Please wait while we load your content...