Controlled synthesis of chiral polymers for the kinetic resolution of racemic amino acids†
Abstract
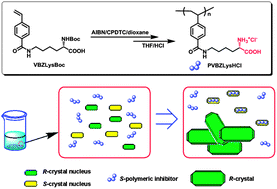
A novel lysine bearing vinyl monomer, (S)-2-(tert-butoxycarbonylamino)-6-(4′-vinylbenzamido)hexanoic acid, was designed and synthesized. It underwent reversible addition–fragmentation chain transfer polymerization induced by azobisisobutyronitrile in the presence of 2-cyano-2-propyl dodecyl trithiocarbonate in dioxane at 65 °C. The number-average molecular weights of the resultant polymers increased linearly with the monomer conversion and the molecular weight distribution indices kept relatively low, indicating a well-controlled chain growth process. After deprotection of N-Boc groups, the monomer and the polymers were used as stereospecific inhibitors in the fractional crystallization of racemic glutamic acid monohydrochloride (rac-Glu·HCl). The polymers showed much better performance than the monomer. At a polymer concentration above 0.5 wt%, the R-enantiomer was obtained with an enantiomeric excess (ee%) over 97%, independent of the molecular weights of the polymer additives. In contrast, 10 wt% of the monomer was required to achieve the same result under identical conditions. However, with an addition of 0.1 wt% polymeric inhibitors, the ee% of the resolved R-Glu·HCl crystals increased initially and then decreased with an increased molecular weight of polymer additives, and for the polymer with a moderate Mn of 5800 Dalton, R-Glu·HCl with ee% over 97% was yielded. Because of the large absorption difference in the UV-Vis range between the polymer inhibitor and the resolved molecules, the residual fraction of additives in the resolved crystals was evaluated by means of UV-Vis spectrophotometry, and all results showed that there were very low fractions of polymer residues (<0.1%). Moreover, the polymers were also efficient for the kinetic resolution of rac-threonine and rac-asparagine monohydrate conglomerates at concentrations of 0.5 and 1.0 wt%, respectively, yielding preferentially R-enantiomers with ee% ∼98%.

 Please wait while we load your content...
Please wait while we load your content...