Renewable β-methylstyrenes for bio-based heat-resistant styrenic copolymers: radical copolymerization enhanced by fluoroalcohol and controlled/living copolymerization by RAFT
Abstract
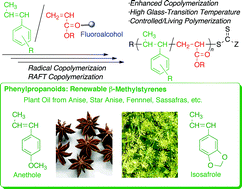
Naturally occurring β-methylstyrenes, such as anethole (Ane), isosafrole (ISa), methyl isoeugenol (MIEu), and acetyl isoeugenol (AcIEu), which are phenylpropanoids and possess electron-donating alkoxy substituents on an aromatic ring, were copolymerized with common acrylic monomers, such as methyl (MA), n-butyl (nBA), and tert-butyl acrylates (tBA), N,N-dimethylacrylamide and diethyl fumarate in toluene and fluoroalcohols. The copolymerizations of β-methylstyrenes (M1) and acrylic monomers (M2) were fitted well by the chain-end model. The relative reactivity of β-methylstyrenes with the acrylic radicals (1/r2) was enhanced up to 3 times in m-C6H4[C(CF3)2OH]2 compared to toluene, whereas no homo-propagation of β-methylstyrenes occurred in any solvent (r1 ∼ 0) because of the sterically hindered 1,2-disubstituted vinyl structure of β-methylstyrenes. The copolymerizability was also dependent on the substituents of both monomers: the relative reactivity of the β-methylstyrenes generally increased with the decreasing bulkiness of both β-methylstyrenes [1/r2 = 1/1.07 (AcIEu/MA), 1/0.62 (MIEu/MA), 1/0.53 (ISa/MA), 1/0.46 (Ane/MA)] (bulkiness: AcIEu > MIEu > Isa > Ane) and acrylates [1/r2 = 1/0.87 (Ane/tBA), 1/0.67 (Ane/nBA), 1/0.46 (Ane/MA)] (bulkiness: tBA > nBA > MA). The reversible addition–fragmentation chain-transfer copolymerization of Ane or ISa with MA proceeded in both toluene and m-C6H4[C(CF3)2OH]2, allowing molecular weight control of the copolymers. The incorporation of β-methylstyrene units into the copolymers was thus increased up to ∼45 mol% (∼60 wt%) by fluoroalcohol, resulting in high-performance polymers with relatively high glass transition temperatures (Tg > 100 °C), even when using common alkyl acrylates as the comonomers.
- This article is part of the themed collection: Sustainable polymers: replacing polymers derived from fossil fuels

 Please wait while we load your content...
Please wait while we load your content...