Polyacetylenes containing BODIPY pendants with different connectivities: synthesis, characterization and opto-electronic properties
Abstract
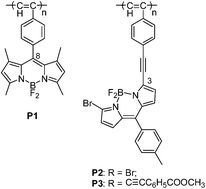
A group of novel polyacetylenes containing BODIPY pendants have been synthesized in satisfactory yield using a [Rh(nbd)Cl]2–Et3N catalyst. The pendant BODIPY unit has been directly conjugated to the polyacetylene backbone either through the 8 position of the BODIPY cores in P1, or through the 3 position in P2 and P3. Their optoelectronic properties have been investigated by UV-vis, FL, CV and Z-scan techniques. Interestingly, the spectroscopy results show that little electronic interaction exists between the BODIPY chromophoric units and the polyacetylene main chain for P1. However, the electronic interaction between the side pendant and the main chain becomes notable for P2 and P3, resulting in a significant broadening and red-shift of their UV-vis absorption and fluorescence emission wavelengths in comparison with those of their respective monomers. The polymers display thermal stabilities and nonlinear optical properties that are dependent on the connectivities of the BODIPY pendants and the polyacetylene backbones. P1 exhibits relatively better thermal stability and poorer nonlinear optical properties than P1 and P2. The third-order nonlinear optical coefficient (χ(3)) of P1 is 8.5 × 10−12 esu, which is shown to be ∼15 times smaller than those of P2 and P3.

 Please wait while we load your content...
Please wait while we load your content...