Synthesis of a cyclen-containing disubstituted polyacetylene with strong green photoluminescence and its application as a sensitive chemosensor towards sulfide anion with good selectivity and high sensitivity†
Abstract
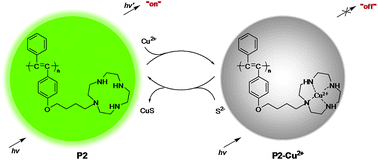
Through a postfunctional method, a new disubstituted polyacetylene (P2) bearing cyclen moieties in the side chains, was prepared conveniently and exhibited strong green fluorescence. P2 showed good pH stability in the solution mixture of THF/H2O (v/v = 1 : 1), and could report the presence of trace Cu2+ ions at a concentration as low as 1.0 × 10−7 mol L−1 in diluted solutions, through an on–off mode. By utilizing the displacement strategy, due to the much higher stability constant of the complex of S2− and Cu2+, the quenched fluorescence of P2 by Cu2+ ions could recover upon the addition of trace S2− anions, with a detection limit down to 2.0 × 10−7 mol L−1. Excitingly, no interference was observed from other anions, including AcO−, SO32−, HSO3−, HCO3−, CO32−, I−, Br−, Cl−, F−, S2O32−, C2O42−, P2O74−, SCN−, HSO4−, SO42−, NO2−, H2PO4−, HPO42−, PO43−, ClO4−, Cr2O72−, S2O82−, ClO3−, IO3−, CN− and NO3−, making P2 a new, sensitive and selective sulfide probe.

 Please wait while we load your content...
Please wait while we load your content...