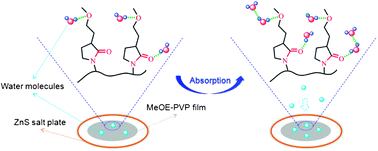
Poly(3-(2-methoxyethyl)-N-vinyl-2-pyrrolidone) (MeOE-PVP), a potential material in the pharmaceutical industry, is an amphiphilic polymer with hydrophobic CH groups and two different hydrophilic groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O), and readily absorbs water vapor from humid environments. In this work, the hydration capabilities and structures of different chemical groups in MeOE-PVP film during water vapor sorption and desorption process are investigated by in situ FTIR spectroscopy, two-dimensional (2D) correlation analysis and density functional theory (DFT) calculations. When the film is tested in a stable humid environment, the changes of the C
O and C–O), and readily absorbs water vapor from humid environments. In this work, the hydration capabilities and structures of different chemical groups in MeOE-PVP film during water vapor sorption and desorption process are investigated by in situ FTIR spectroscopy, two-dimensional (2D) correlation analysis and density functional theory (DFT) calculations. When the film is tested in a stable humid environment, the changes of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group are more sensitive to water vapor than those of the C–O group, which is further verified by 2DIR maps, with much more hydrated structures of the C
O group are more sensitive to water vapor than those of the C–O group, which is further verified by 2DIR maps, with much more hydrated structures of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group being formed after moisture absorption. Compared with MeOE-PVP in aqueous solution, it is believed that C–O group is a relatively stable hydrophilic group from which is difficult to lose water molecules once it is at the hydrated state. In addition, combined with DFT calculations, it is observed that C
O group being formed after moisture absorption. Compared with MeOE-PVP in aqueous solution, it is believed that C–O group is a relatively stable hydrophilic group from which is difficult to lose water molecules once it is at the hydrated state. In addition, combined with DFT calculations, it is observed that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O groups of MeOE-PVP are inclined to interact with water molecules in the form of CO⋯H2O and CO⋯H2O⋯H2O, but as CO⋯(H2O)2, probably due to limited absorption concentration and contact area in the MeOE-PVP film.
O and C–O groups of MeOE-PVP are inclined to interact with water molecules in the form of CO⋯H2O and CO⋯H2O⋯H2O, but as CO⋯(H2O)2, probably due to limited absorption concentration and contact area in the MeOE-PVP film.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O), and readily absorbs
O and C–O), and readily absorbs ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group are more sensitive to
O group are more sensitive to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group being formed after moisture absorption. Compared with MeOE-PVP in aqueous solution, it is believed that C–O group is a relatively stable hydrophilic group from which is difficult to lose
O group being formed after moisture absorption. Compared with MeOE-PVP in aqueous solution, it is believed that C–O group is a relatively stable hydrophilic group from which is difficult to lose ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O groups of MeOE-PVP are inclined to interact with
O and C–O groups of MeOE-PVP are inclined to interact with 

 Please wait while we load your content...
Please wait while we load your content...