Well-defined temperature-sensitive surfactants for controlled emulsion coalescence†
Abstract
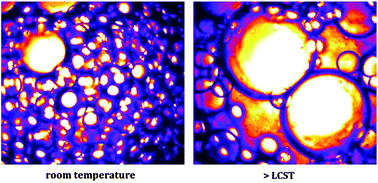
In a variety of applications, emulsion formulations are required, which exhibit excellent shelf stability yet can be broken or perform phase inversion at a desired time. Here we approach these contradictory constraints through the synthesis of well-defined thermoresponsive surfactants based on di(ethylene glycol)methacrylate and poly(ethylene glycol)methacrylate using Atom Transfer Radical Polymerization. The surfactants show a Lower Critical Solution Temperature (LCST) of approximately 34 °C, independent of molecular weight, which is ascertained by both Differential Scanning Calorimetry as well as Dynamic Light Scattering. Below the LCST, the surfactants stabilize the emulsions for at least four months. Above this temperature the hydrophilic block collapses and coalescence between the emulsion droplets occurs; this leads to demixing of the sample within several minutes. We reveal the mechanism for the temperature-triggered coalescence by measurements of the temperature-dependent interfacial tension and by studying the interfacial morphology of surfactant-covered emulsion droplets.

 Please wait while we load your content...
Please wait while we load your content...