Bioinspired phospholipid polymer prodrug as a pH-responsive drug delivery system for cancer therapy
Abstract
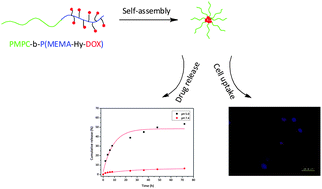
Efficient delivery systems should be stable in blood circulation, with efficient cellular uptake and rapid drug release in cancer cells. Herein, we synthesized P(2-(methacryloyloxy)-ethyl phosphorylcholine)-b-P(2-methoxy-2-oxoethyl methacrylate) via atom transfer radical polymerization. Doxorubicin (DOX) was linked to the polymer via a pH-responsive hydrazone bond. The polymer prodrug had high DOX content (10.6 wt%) and was able to self-assemble to form core–shell structured micelles. Dynamic light scattering showed that the average size of the micelles was 142.3 nm, which is the ideal size for the enhanced permeability and retention (EPR) effect. The shell of the micelles was composed of phosphorylcholine, which imitated the structure of cell membranes. Studies of intracellular uptake demonstrated that the prodrug micelles were internalized effectively by cancer cells. An in vitro release study indicated that the release of DOX at pH 5.0 was much faster than that at pH 7.4. Moreover, in vitro cytotoxicity showed that this polymer prodrug inhibited the growth of cancer cells remarkably, demonstrating its potential for use as an efficient drug delivery system.

 Please wait while we load your content...
Please wait while we load your content...