Synthesis of hyperbranched polythiophene with a controlled degree of branching viacatalyst-transfer Suzuki–Miyaura coupling reaction
Abstract
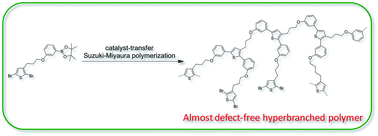
A hyperbranched polythiophene with the degree of branching of almost 100% has been successfully prepared by the

 Please wait while we load your content...
Please wait while we load your content...