Aza-BODIPY-based D–π–A conjugated polymers with tunable band gap: synthesis and near-infrared emission†
Abstract
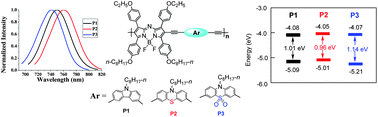
Three novel donor–π–acceptor (D–π–A) type polymers P1, P2 and P3 could be synthesized from a diiodo substituted aza-borondipyrromethene (aza-BODIPY) derivative (M-1) with 3,6-diethynyl-9-octyl-9H-carbazole (M-2), 3,7-diethynyl-10-octyl-10H-phenothiazine (M-3) and 3,7-diethynyl-10-octyl-10H-phenothiazine-S,S-dioxide (M-4) via a Pd-catalyzed Sonogashira coupling reaction. These resulting aza-BODIPY-based conjugated polymers show narrow near-infrared emission in the range of 742–763 nm. The D–π–A polymers can also exhibit an interesting trend of visible color (cyan for P1, green for P2 and light green for P3) in solution by the choice of different monomer structures. The band gaps of the alternating polymers can be tuned in the range 0.96–1.14 eV by using three different donors, which can be attributed to the internal charge transfer from an electron-rich to an electron-deficient moiety.

 Please wait while we load your content...
Please wait while we load your content...