Synthesis and characterization of polythiophene grafted with a nitroxide radical polymer via atom transfer radical polymerization
Abstract
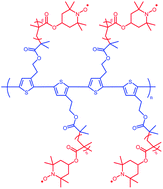
Synthesis of 2,5-poly(3-[1-ethyl-2-(2-bromoisobutyrate)]thiophene)-graft-poly(2,2,6,6-tetramethylpiperidin-1-oxyl-4-yl methacrylate) (PEBBT-g-PTMA) and its electrochemical properties in organic radical batteries have been reported. The polythiophene-based macroinitiator PEBBT, synthesized from 3-[1-ethyl-2-(2-bromoisobutyrate)]thiophene, grafts with poly(2,2,6,6-tetramethylpiperidin-4-yl methacrylate) (PTMPM) to yield PEBBT-g-PTMPM via atom transfer radical polymerization (ATRP). The conditions for ATRP of 2,2,6,6-tetramethylpiperidin-4-yl methacrylate (TMPM) are optimized by examining a series of model polymerizations. PEBBT-g-PTMPM is oxidized by m-chloroperoxybenzoic acid to yield PEBBT-g-PTMA with a relatively high molecular weight (Mn = 483 300) that prevents the dissolution of the polymer into electrolytes. PEBBT-g-PTMA has been evaluated as a cathode-active material in a rechargeable organic radical battery. The result of cyclic voltammetry shows a redox couple at approximately 3.6 V (vs. Li/Li+). Furthermore, the cell performance shows that the organic radical battery has good electrochemical stability and good cyclability.

 Please wait while we load your content...
Please wait while we load your content...