Properties and degradation of hydrocarbon fuel cell membranes: a comparative study of sulfonated poly(arylene ether sulfone)s with different positions of the acid groups†
Abstract
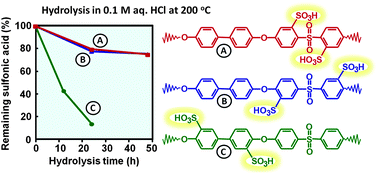
Sulfonated fully aromatic polymers with different positions of the acid groups, but with an identical polymer backbone, have been investigated and compared with respect to their properties as proton-exchange membranes. Three different series of sulfonated poly(arylene ether sulfone)s (SPAES) having the same backbone structure were prepared from 4,4′-dichlorodiphenyl sulfone (DCDPS) and 4,4′-dihydroxybiphenyl (BP), each series comprising three levels of the ion exchange capacity (IEC). The first series of SPAES had the sulfonic acid groups placed in ortho positions to the ether bridges (oeSPAES) and were prepared through post-polymerization sulfonation. The second and third series of SPAES carried the sulfonic acid groups in meta (msSPAES) and ortho (osSPAES) positions to the sulfone bridges, respectively, and were prepared by polycondensations of mixtures of BP, DCDPS and either 3,3′-disulfonated or 2,2′-disulfonated DCDPS, respectively. The latter monomer was synthesized via lithiation–sulfination–oxidation of DCDPS. Analysis of solvent cast membranes showed that the osSPAES copolymers had a high dimensional stability in water up to 100 °C, even at high ionic contents. Moreover, the osSPAES and the msSPAES membranes were better at retaining conductivity at reduced relative humidity than the oeSPAES membranes. Thermogravimetry of the membranes in the acid form under air indicated no significant differences based on the placement of the sulfonic acid groups. The resistance towards radical attack was analyzed by 1H NMR spectroscopy after immersing the membranes in Fenton's reagent at 60 °C, and the ability to retain IEC was found to increase in the order oeSPAES < msSPAES < osSPAES. Membrane stability was also studied in an accelerated hydrolysis test by immersing the membranes in 0.1 M aq. HCl at 200 °C in a sealed vessel. The results showed a dramatic loss of sulfonic acid in the oeSPAES membranes, presumably activated by the proximity of the ether bridges. Careful 1H NMR analysis also suggested that the osSPAES polymers degraded by scission of the sulfone bridges. The position of the sulfonic acid groups ortho to the sulfone bridges seemingly destabilized the latter under the severe conditions employed in the test.

 Please wait while we load your content...
Please wait while we load your content...