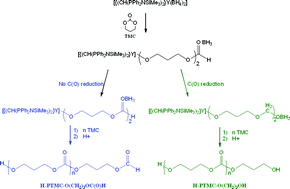
Bis(phosphinimino)methanide bisborohydride complexes of lanthanum, yttrium and lutetium, [{CH(PPh2NSiMe3)2}La(BH4)2(THF)] (1) and [{CH(PPh2NSiMe3)2}Ln(BH4)2] (Ln = Y (2), Lu (3)), have been investigated in the ring-opening polymerization (ROP) of trimethylene carbonate (TMC). All three initiators afforded linear poly(trimethylene carbonate)s (PTMCs) in toluene at 23 °C. 1H NMR analyses of the polycarbonates revealed the formation of α-hydroxy,ω-formate telechelic PTMCs, as previously observed in the ROP of TMC initiated by [Sm(BH4)3(THF)3]. This suggested the non-reduction of the carbonyl of the carbonate group in the active species by the BH3 moiety, as hinted by this same prior experimental work. Formation of α,ω-dihydroxy PTMCs, resulting from the reduction of the carbonyl, is also likely and cannot be ruled out from experimental data. DFT investigations, focused on the initiation step, supported two energetically (thermodynamically and kinetically) favorable and similar reaction pathways leading to two distinct end-functionalized PTMCs. Depending on whether reduction of the carbonyl occurred or not, α,ω-dihydroxy or α-hydroxy,ω-formate telechelic PTMCs were predicted, respectively. Although these two calculated feasible approaches are very close in energy, the formation of the latter heterofunctionalized α-hydroxy,ω-formate telechelic PTMCs is slightly preferred computationally. This first in silico study on the mechanism of the ROP of a cyclic carbonate revealed several features without any precedent in the ROP of a cyclic ester (ε-caprolactone or lactide). An easily accessible (no activation barrier) intermediate in which BH3 is trapped by the intracyclic oxygen of a non-opened TMC ring has been located for the first time. The low activation barrier for the opening of the TMC ring that proceeds without B–H activation is predicted to be competitive, affording the thermodynamically less stable BH3 adduct. Finally, trapping of BH3 by the nitrogen of the {CH(PPh2NSiMe3)2}− ligand, in agreement with previous findings, highlights again the valuable role of this bisphosphiniminomethanide ligand.

 Please wait while we load your content...
Please wait while we load your content...