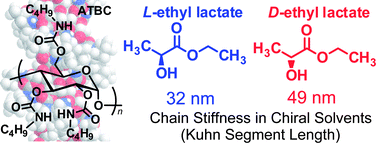
The z-average radius of gyration, the particle scattering function and the intrinsic viscosity have been determined for amylose tris(n-butylcarbamate) (ATBC) in D-, DL- and L-ethyl lactates, all at 25 °C, by light and small-angle X-ray scattering and viscometry as functions of the weight-average molecular weight ranging from 1.7 × 104 to 1.7 × 106 to investigate the chiral solvent dependence of the helical conformation of the amylose derivative. The data were analyzed in terms of the wormlike chain model to determine the Kuhn segment length λ−1 (the stiffness parameter) and the helix pitch per residue h. The λ−1 value of 49 nm in D-EL is 52% larger than 32 nm in L-ethyl lactate, an unmistakable chiral solvent effect to the helical conformation of the amylose derivative, and the relation between h and λ−1 is consistent with that obtained previously in nine different solvents [Polymer, 2010, 51, 4243]. The content of intramolecularly hydrogen bonding C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in D-ethyl lactate is estimated to be 15% more than that in L-ethyl lactate, confirmed by heats of dilution for ethyl lactate solutions of ATBC estimated by isothermal titration calorimetry.
O groups in D-ethyl lactate is estimated to be 15% more than that in L-ethyl lactate, confirmed by heats of dilution for ethyl lactate solutions of ATBC estimated by isothermal titration calorimetry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in D-ethyl lactate is estimated to be 15% more than that in
O groups in D-ethyl lactate is estimated to be 15% more than that in 

 Please wait while we load your content...
Please wait while we load your content...