Multifunctional and biodegradable polyphosphazenes for use as macromolecular anti-cancer drug carriers†
Abstract
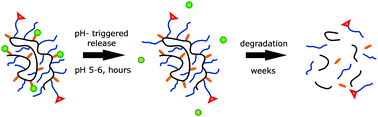
Using a living cationic polymerisation procedure we synthesised a series of multi-armed poly(organo)phosphazenes with controlled molecular weights and excellent aqueous solubility. The synthetic flexibility of polyphosphazenes was exploited in order to incorporate an acid-sensitive hydrazide linker to the polymer backbone, as well as tumour-targeting folic acid groups. We were then able to attach hydrophobic anti-cancer

 Please wait while we load your content...
Please wait while we load your content...