The interaction of C-terminal Tyr208 and Tyr13 of the first α-helix ensures a closed conformation of ctenophore photoprotein berovin
Abstract
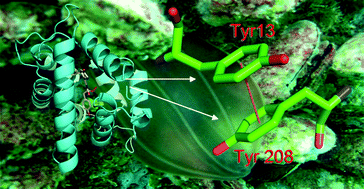
Light-sensitive Ca2+-regulated photoprotein berovin is responsible for the bioluminescence of the ctenophore Beroe abyssicola. It shares many properties of hydromedusan photoproteins although the degree of identity of its amino acid sequence with those of photoproteins is low. There is a hydrogen bond between C-terminal Pro and Arg situated in the N-terminal α-helix of hydromedusan photoproteins that supports a closed conformation of the internal cavity of the photoprotein molecule with bound 2-hydroperoxycoelenterazine. The C- and N-terminal hydrogen bond network is necessary to properly isolate the photoprotein active site from the solvent and consequently to provide a high quantum yield of the bioluminescence reaction. In order to find out which berovin residues perform the same function we modified the N- and C-termini of the protein by replacing or deleting various amino acid residues. The studies on berovin mutants showed that the interaction between C-terminal Tyr208 and Tyr13 localized in the first α-helix of the photoprotein is important for the stabilization and proper orientation of the oxygenated coelenterazine adduct within the internal cavity as well as for supporting the closed photoprotein conformation. We also suggest that the interplay between Tyr residues in ctenophore photoproteins occurs rather through the π–π interaction of their phenyl rings than through hydrogen bonds as in hydromedusan photoproteins.


 Please wait while we load your content...
Please wait while we load your content...