Dimerization of LOV domains of Rhodobacter sphaeroides (RsLOV) studied with FRET and stopped-flow experiments
Abstract
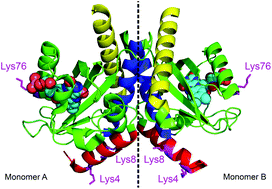
The bacterium Rhodobacter sphaeroides has a short LOV (light-oxygen-voltage) domain, which is not connected to an effector domain but has an α-helix extension at the N-terminus as well as a helix-turn-helix (HTH) motiv at the C-terminus. These extensions offer possibilities for interactions with effector enzymes or DNA. Whereas many LOV domains show a tendency to form dimers in the light state, RsLOV is unique in that it is a dimer in the dark state but dissociates into monomers after blue-light excitation. We studied the kinetics of this dimerization process by a combination of FRET spectroscopy and stopped-flow experiments with a time resolution of ≈10 ms. Although excitation of the flavin chromophore in dye-labeled LOV domains leads to considerable FRET from flavin to the dye, the typical adduct formation between flavin and a nearby cysteine still occurs with considerable yield. We obtain a rate constant for LOV–LOV dimerization in the range (0.8–1.8) × 105 M−1 s−1, and an equilibrium constant of the dark-state dimer in the range (3.0–7.0) × 10–6 M. Dissociation of the dimers in the light state and reforming of dimers after return to the dark state was monitored using an anti-FRET effect caused by excitonic interaction between dye labels on different monomers. Reforming of the dark state dimers is slower than recovery of the flavin–cysteinyl adduct, indicating that light-induced conformational changes in the LOV domain persist for much longer time than the adduct lifetime.
- This article is part of the themed collection: Photofunctional flavoproteins and UVB photosensors


 Please wait while we load your content...
Please wait while we load your content...