The photoisomerization of cis,trans-1,2-dideuterio-1,4-diphenyl-1,3-butadiene in solution. No bicycle-pedal†
Abstract
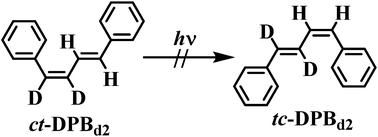
cis,trans-1,2-Dideuterio-1,4-diphenyl-1,3-butadiene (ct-DPBd2) was synthesized and its cis–trans photoisomerization in cyclohexane-d12 (C6D12) at room temperature was monitored by 1H NMR spectroscopy. The results reveal formation of only trans,trans-1,2-dideuterio-1,4-diphenyl-1,3-butadiene (tt-DPBd2). The failure to detect formation of trans,cis-1,2-dideuterio-1,4-diphenyl-1,3-butadiene (tc-DPBd2) eliminates the possibility that an identity bicycle pedal process contributes to inefficiency in the cis–trans photoisomerization of cis,trans-1,4-diphenyl-1,3-butadiene (ct-DPB).
- This article is part of the themed collection: In memory of Professor Ugo Mazzucato (1929–2017)


 Please wait while we load your content...
Please wait while we load your content...
