Abstract
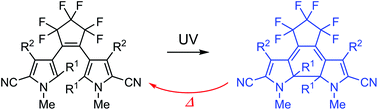
Although diarylethene derivatives are considered to undergo thermally irreversible photochromic reactions, this is not always the case. Here, we report on thermally reversible photochromic diarylethenes having pyrrole-2-carbonitrile aryl groups. The thermal stability of the coloured closed-ring isomer of 1,2-bis(2-alkyl-5-cyano-1-methyl-3-pyrrolyl)perfluorocyclopentene was found to depend on alkyl substituents at the 2- and 2′-positions. The closed-ring isomer of the ethyl-substituted derivative thermally returned back to the open-ring isomer much faster than that of the methyl-substituted derivative. The difference in the thermal stability was well explained by the ground state energy difference between open- and closed-ring isomers. Excellent fatigue resistance and appropriate thermal fading rates were observed for the derivative having cyano substituents at the 5- and 5′-positions and methyl substituents at the 2-, 2′-, 4- and 4′-positions. The UV-irradiated colouration and thermal decolouration cycle can be repeated more than 103 times.
- This article is part of the themed collection: In memory of Professor Ugo Mazzucato (1929–2017)


 Please wait while we load your content...
Please wait while we load your content...