Novel photoresponsive cyclicparaphenylenediazenes: structure, strain energy, cis–trans isomerization, and electronic properties†
Abstract
A series of cis–trans isomers of cyclicparaphenylenediazenes (CPPDs) have been designed to explore their potential applications in solar thermal fuels and photoswitchable devices. In this work, three isomers of cis–trans-[3]CPPD, seven isomers of cis–trans-[4]CPPD, eleven isomers of cis–trans-[5]CPPD, and sixteen isomers of cis–trans-[6]CPPD have been proposed using density functional theory (DFT) at the B3LYP/6-31+G(d,p) level of theory. The stability of these CPPDs has been quantified by the homodesmotic reaction approach. Strain energies (SE) indicate that 3-cct, 4-ctct-anti, 5-cctct-anti, and 6-cttttc-anti are stable molecules in their respective CPPDs. The SE and heats of formation 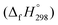 of cis–trans-CPPDs were also compared with those of all-cis-CPPDs and all-trans-CPPD isomers. The calculations suggest that cis–trans-CPPDs are more stable than all-cis and all-trans-CPPDs. The SE and
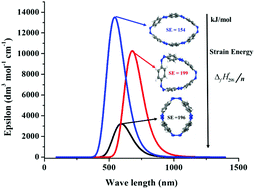
of cis–trans-CPPDs were also compared with those of all-cis-CPPDs and all-trans-CPPD isomers. The calculations suggest that cis–trans-CPPDs are more stable than all-cis and all-trans-CPPDs. The SE and 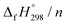 also suggest that 3-cct, 4-ctct-anti, 5-cctct-anti, and 6-cttttc-anti are important candidates for laboratory test. The calculated highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) energy gaps of cis–trans-CPPDs indicate that these oligomers are potential materials for the construction of solar cells. Time-dependent (TD) DFT calculations of CPPDs show a characteristic peak in the range of 450 nm to 600 nm, which is consistent with previous studies. The predicted structures, and thermochemical and electronic properties can be a good starting point for the synthesis of CPPD-based photoswitchable and solar fuel cell devices.
also suggest that 3-cct, 4-ctct-anti, 5-cctct-anti, and 6-cttttc-anti are important candidates for laboratory test. The calculated highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) energy gaps of cis–trans-CPPDs indicate that these oligomers are potential materials for the construction of solar cells. Time-dependent (TD) DFT calculations of CPPDs show a characteristic peak in the range of 450 nm to 600 nm, which is consistent with previous studies. The predicted structures, and thermochemical and electronic properties can be a good starting point for the synthesis of CPPD-based photoswitchable and solar fuel cell devices.


 Please wait while we load your content...
Please wait while we load your content...