Photophysics of perylene monoimide-labelled organocatalysts†
Abstract
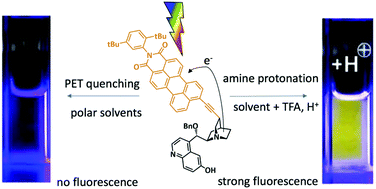
We designed and synthesized cinchona alkaloid derivates PMI-BnCPD, 1 and PMI-dHQD, 2, in which a fluorescent perylene monoimide unit is linked to the quinuclidine fragment. The latter acts as an electron donor, quenching the perylene imide fluorescence in polar solvents. In the organocatalytic application of these compounds, the electron donor is deactivated by binding to an electrophile, e.g. H+. We show that this restores the fluorescence, allowing the compounds to signal the electrophile binding step that occurs in many catalytic reactions. In order to demonstrate that charge transfer is indeed the fluorescence quenching mechanism, we detected the charge separated state by means of transient absorption spectroscopy. Incidentally, the excited state absorption bands of the locally excited and charge transfer states are very similar. The activity of the fluorophore labeled organocatalyst 1 in a fluorogenic Michael addition reaction is demonstrated.


 Please wait while we load your content...
Please wait while we load your content...