The electronic transitions of analogs of red wine pyranoanthocyanin pigments†
Abstract
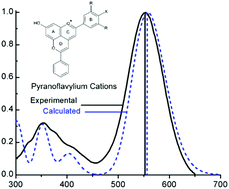
There is increasing interest in using natural colorants like anthocyanins in cosmetics, food and pharmaceuticals as replacements for synthetic colorants. During the maturation of red wines, the anthocyanin pigments contained in grapes are transformed via reaction with copigments and metabolic products into pyranoanthocyanins, responsible in part for the final color of the wine. In order to understand structural effects on the absorption spectra of pyranoanthocyanins, the calculated excited state energies and spectroscopic states of a series of substitued pyranoflavylium cation analogs of pyranoanthocyanins have been compared to experimental spectroscopic data for these compounds. The vertical excitation energies, calculated by using the ADC(2) approach, gave excellent agreement with the experimental UV-Vis spectra and the nature of the lowest excited state correlates with the observed photophysical behavior in solution. The present results thus provide a basis for the design of new pyranoflavylium chromophores with the desired colors and photophysics, as well as for understanding the analogous properties of natural pyranoanthocyanin pigments in red wine.


 Please wait while we load your content...
Please wait while we load your content...