Effects of substituents in silyl groups on the absorption, fluorescence and structural properties of 1,3,6,8-tetrasilylpyrenes
Abstract
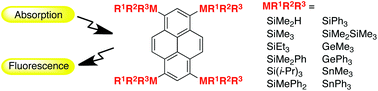
1,3,6,8-Tetrasilylpyrenes and related germyl and stannyl derivatives were synthesized, and their absorption and fluorescence spectroscopic and structural properties were elucidated. The results show that the UV-vis absorption maxima of these substances in CH2Cl2 solutions shift to longer wavelengths as the size of the alkyl groups and numbers of phenyl groups on silicon increase. Fluorescence quantum yields of tetrasilylpyrenes in cyclohexane are larger than that of pyrene, and a pentamethyldisilyl derivative has an emission efficiency of 0.79. Except in the case of the SiMe2H derivative, excimer emission was not observed in concentrated solutions of these substances. The SiMe2H and SiMe3 derivatives were shown to form CT complexes with tetracyanoethylene in CH2Cl2 solutions. The calculated energy barriers for rotation of the silyl groups about the Si–C bond increase as the steric bulk of the silyl group increases. 29Si NMR chemical shifts were found to depend on the sizes of the alkyl groups and numbers of phenyl groups. Data arising from theoretical calculations suggest that the silyl groups act as electron-donating groups, and the donating ability of the groups decreases in the order SiR3 > GeR3 > SnR3.


 Please wait while we load your content...
Please wait while we load your content...
